
South Korea’s SK Biopharmaceuticals Co. has signed a deal with Brazil’s Eurofarma Laboratórios S.A. to set up a joint venture in the US to develop an AI-based epilepsy treatment platform.
The drug-developing subsidiary of Korea’s No. 2 conglomerate SK Group said it plans to hire local executives, implement localization strategies and leverage its existing US network built through direct sales of SK Biopharma’s flagship antiepileptic drug Cenobamate.
SK Biopharma Chief Executive Lee Donghoon announced the plan at a press conference on Monday in San Francisco, on the sidelines of the JP Morgan Healthcare Conference.

“We first connected with Eurofarma at the AES (American Epilepsy Society) three years ago, and this partnership has come to represent a new chapter in SK Biopharma’s global expansion,” he said. “Through this joint venture, we aim to become the No. 1 AI-based epilepsy management solutions provider in North America.”
In July 2022, SK Biopharma signed a deal to license out its technology for Cenobamate to Eurofarma in order to enter the Latin American market.
Under the deal, SK Biopharmaceuticals will give Eurofarma exclusive rights to manufacture and commercialize Cenobamate in Latin America.
With the technology transfer, SK said it aims to sell Cenobamate throughout Latin America via its Brazilian partner.
Eurofarma has been operating in the healthcare industry since its establishment in 1972.
TELE-EPILEPSY MARKET
The global digital health market is expected to grow at 25% on average annually over the next few years, with significant investments being directed toward AI-driven diagnostics, prevention and management.
The primary focus of the JV will be tele-epilepsy treatment – a market that SK Biopharma projects to grow to $1.8 billion by 2032, with North America accounting for 47% of the global market.
The JV will leverage SK Biopharma’s AI technology for electroencephalogram (EEG) analysis and wearable EEG monitoring devices.
SK said the technology forms the basis for AI-powered epilepsy management solutions, which will enable real-time monitoring of seizures, support optimal treatment plans through data-driven insights and enhance communication between patients and healthcare providers.

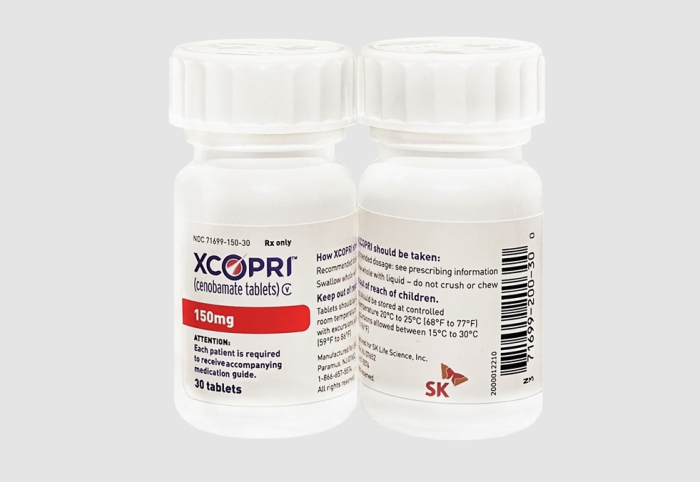
In May 2020, SK began selling Cenobamate, a medication used for the treatment of partial onset epileptic seizures, in the US under the brand name Xcopri.
Cenobamate is the first drug that SK has developed on its own from candidate substances discovery to clinical trials, licensing and sales in the US after obtaining approval from the Food and Drug Administration.
Epilepsy is one of the most common neurological diseases, affecting over 6 million people in Latin America alone.
“While many IT companies venture into the biotech space, they often face limitations due to a lack of clinical data and field experience. We aim to become a pharmaceutical company that offers complete solutions from medical devices to drugs. By integrating clinical data into digital platforms, we will develop AI models to predict abrupt epileptic seizures,” Lee said.
SK Biopharmaceuticals aims to become one of the world’s top 10 healthcare companies by 2030 with an enterprise value of 50 trillion won ($44 billion) by then.
By Young-Ae Lee
0ae@hankyung.com
In-Soo Nam edited this article.















